Congruence Medical Solutions LLC
The Viscous Dosing Syringe (VDS) platform reduces the manual force required to inject, thereby enabling users to more comfortably inject highly viscous drugs (up to 3000cP), as well as cold drugs and drugs requiring a fine injection needle.
It is easy to use and includes a proprietary integrated passive needle safety technology, making it suitable for use by a clinician, caregiver or patient (self-injection). This versatile platform incorporates standard pre-fillable syringes and has a wide array of customization options.

- Disposable, Single-Use, Single-Dose (0.01mL – 2.25mL)
- User controls injection speed
- Dose setting options: Pre-set or User-set
- Drug filling options: Pre-filled or User-filled (from a vial)
- All options incorporate standard, third-party, pre-fillable ISO 11040 compliant syringes
- No user-assembly required
Key Benefits
- High accuracy: within 5% of target dose
- Easy to Use
- Controlled Priming & unlocking of plunger rod in one simple step
- One-handed operation possible
- Can deliver highly viscous drugs with up to 75% reduced injection force (vs standard syringe1)
- User Preferred (90% users prefer VDS vs syringe1)
- Reduces drug waste
Availability
- IP Protected
- Samples available for clinical/preclinical use
Applications
The VDS is suitable for applications where the injection force is high.
This is the case with viscous drugs, as viscosity has a direct impact on injection force, making it hard in some cases for users to manually depress the plunger rod.
Pharmaceutical formulations are increasingly becoming viscous due to trends towards more high strength biological therapeutics and/or long-acting formulations. Viscosity can range from 10-150cP concentrated biologic formulations found in oncology, autoimmune disorders, ophthalmology, and other areas, and several long-acting formulations have even higher viscosities (1000cP+).
Pharmaceutical companies often have to increase injection volume to offset effect of higher formulation viscosity, which leads them to require more than one injection device for a complete dose or use expensive larger volume injection platforms such as patch pumps or even consider IV therapy / infusions. The Congruence VDS is a suitable alternative to these expensive and/or less user-friendly options.
Other application areas can include drugs that are refrigerated, as at lower temperatures injectable therapies can become more viscous, raising the possibility of users experiencing high injection forces if the drug is injected too soon after refrigeration removal. Drugs that are delivered through a fine needle gauge will experience higher injection forces, so the VDS is also applicable there.

Customize
Key configuration choices are:
- Drug Filling: Pre-filled (syringe is primary drug container) or User-filled (Vial is primary drug container)
- Dose Setting: Pre-set (factory-set) or User-Set (Multiple Dose volume options)
Note: In order to ensure highest quality drug contact materials, the VDS incorporates pre-fillable syringes even if the drug is not prefilled in the syringe
Other customization options include:
- Choice of various ISO 11040 compliant standard syringes:
- Siliconized, silicone-free or lubrication free
- 0.5mL, 1mL long, 1mL short, 2.25mL, 5mL; Glass or polymer
- Luer-lock or staked needle
- Accommodation for wide range of drug viscosities (up to 3000cP), specific injection volume(s), target injection time and preferred needle gauge
- External look and feel
- Start and/or End of Dose indicators (audible, visual)
- Other based on route of administration
If you are interested in other customization and configuration options to meet your specific needs, please contact us.
Data
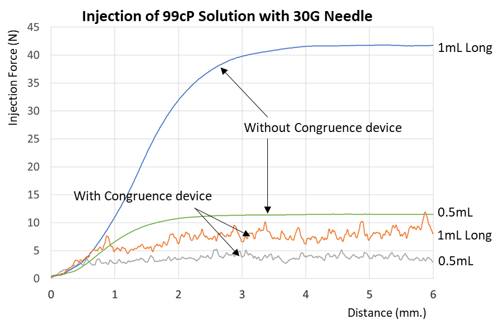
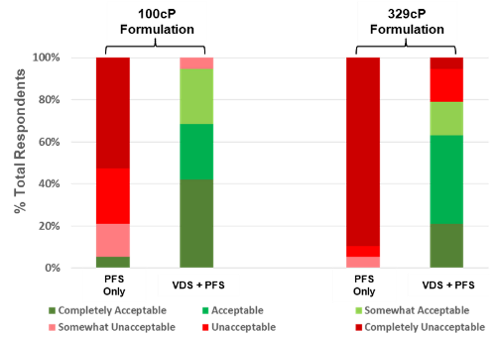
Congruence devices have been shown to inject high viscosity formulations with significantly low force from the user compared to a standard pre-fillable syringe [PFS] alone even when incorporating the same PFS.
The reduction in required user force results in much higher user acceptance in comparison to PFS. Indeed, 100% of the clinicians in a Congruence user study preferred the Congruence VDS device over only the PFS.
The VDS platform was featured in a presentation at PODD 2021.
Additional data available upon request
Specifications
| Key Needs Addressed | Microliter-Milliliter, (Highly) Viscous |
| Device Type | Manual Syringe Injector |
| User Profile | HCPs, Caregivers, Patients |
| Administration Route | Subcutaneous, Intramuscular |
| Primary Container | 0.5mL, 1mL long, 1mL short or 2.25mL standard prefilled glass syringe; (Vial for user-filled) |
| Usage | Single-Dose; Disposable |
| Dosage Type | Fixed or Variable |
| Dose Volume | 0.01mL – 2.25mL |
| Viscosity | Up to 3000cP |
| Injection Time | User Controlled |
| Priming & Dose Setting | Simple Dial Turn (combined user step) |
| Needle Attachment | User attached or Pre-attached |
| Needle Safety | Integrated Passive Needle Shield |
| Injection Feedback | Drug Viewing Window, Visual and Audible dose indicators |
Instructions for use available upon request
