Congruence Medical Solutions LLC
About Us
Purpose
At Congruence, our purpose is to enhance the clinical and commercial impact of injectable drugs that have unsolved, emerging and hard-to-address delivery needs.
The world of injectable drugs is continually evolving, growing and diversifying. These changes continually generate new, compelling drug delivery needs. However, many incumbent drug delivery device companies are not structured or focused in a way that allows them to identify and address these needs adequately. In short, delivery device development has often lagged advances in pharmaceutical science, meaning pharma and biotech companies have sometimes adopted suboptimal delivery solutions for critical therapies.
Our goal is to address this gap. We want to be the company that pharmas and biotechs turn to when they need a knowledgeable device partner that has proactively developed innovative, practical platform solutions. We also want to be responsive, agile and humble enough to continue to anticipate and innovate to address their evolving needs.

Congruence HQ in Baltimore, MD
Values and Capabilities
We have purposefully built systems, processes and a culture that enable us to be forward-looking, innovative, practical, nimble and responsive, while also ensuring we always strive to act with integrity and in congruence with our customers, community and environment.
We design, develop and supply our drug delivery devices. To do this, we leverage core capabilities that include:
- Deep Industry Knowledge
- User Informed Development
- Human Factors Engineering
- Robust Development Process
- Rapid Prototyping
- Testing and Design Verification
- Design for Manufacturing
- Regulatory and Compliance Expertise
- Quality System
- Intellectual Property
- Versatile Product Platforms
- Strong Industry Network
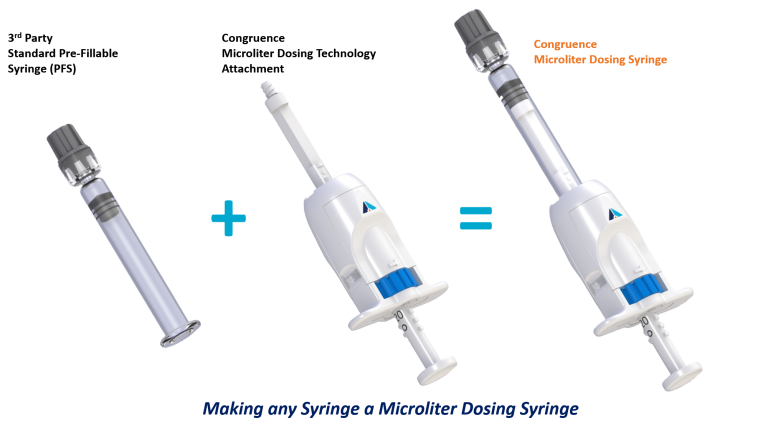
Our devices incorporate standard primary containers, such as third-party pre-fillable syringes. Our innovations solve drug delivery problems by “adding value” to these standard primary containers without compromising the drug-container closure. For example, we take a standard syringe and by adding our Microliter Dosing attachment to it, we convert it into a Microliter Dosing Syringe, that can (amongst other benefits) deliver microliter doses much more accurately.
We are happy to work with the primary containers our customers have already selected, or to work with them to find a suitable vendor in our industry-proven, expert network. The same applies to manufacturing options. We believe offering this flexibility enables us to meet our customers’ needs.
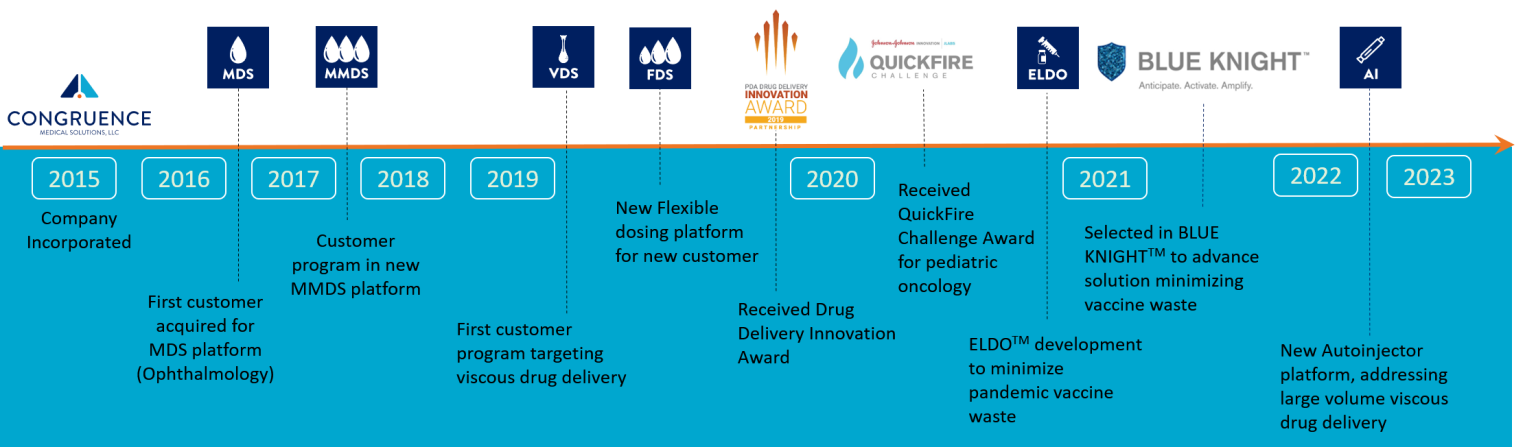
History